New Product: 45W/65W USB-C Medical/ITE GaN PD Charger - EUM106AXR Series

Introduction
To address the increasing issue of electronic waste from various devices and their incompatible chargers, the European Union enacted legislation on November 23, 2022, mandating that, starting December 28, 2024, all mobile phones, tablets, cameras, gaming consoles, headphones, and other handheld electronic devices must adopt the USB-C (Type C) charging interface. This change will enable consumers to charge multiple devices with a single charger and Type-C cable. While the regulation is expected to have a profound impact on the environment, the electronics industry, and consumers, it would also affect the medical industry.
Many medical devices, especially portable ones such as monitoring equipment, handheld diagnostic tools, and wearable health devices, could transition to using USB-C for power and data transfer. This would simplify the logistics of managing and maintaining these devices, as healthcare facilities would need fewer types of chargers and cables, leading to reduced costs and easier inventory management. With a standardized charging interface, the compatibility between different medical devices could improve.
This could make it easier to integrate new devices into existing systems, reducing the time and effort required for setup and ensuring that devices can be quickly replaced or upgraded without worrying about compatibility issues. Also, the use of a universal charging interface like USB-C enhances portability. It would make it more convenient to charge the devices on the go, ensuring they are always ready for use, which is critical in emergency situations. The medical industry is not immune to the challenges of electronic waste. By adopting USB-C, the industry could contribute to reducing electronic waste, aligning with broader sustainability goals and regulatory requirements.
In response to the existing circumstances mentioned above, EDAC POWER is excited to introduce the EUM106AXR series, our latest design to meet both Medical and ITE standards, after the launch of EU204AS(45W) and EU306AS(65W) ITE PD GaN Charger. EUM106AXR builds on the strengths of its predecessor, offering enhanced features and compliance with medical standards, making it the perfect solution for a wide range of applications in medical healthcare and information technology.
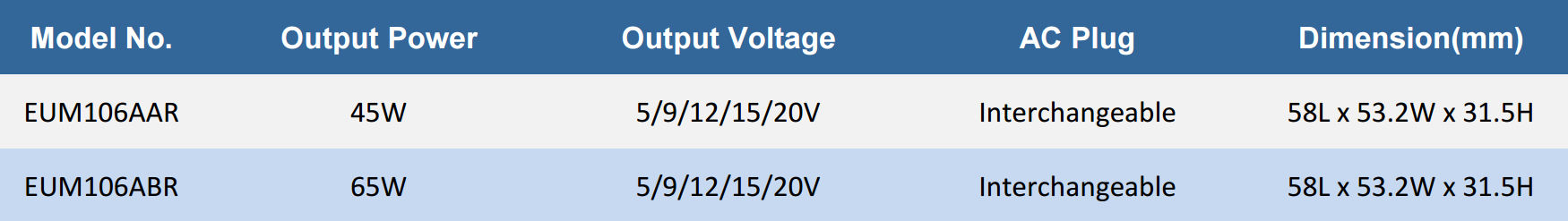
EUM106AXR series is available in both 45W and 65W output options, equipped with USB Power Delivery (PD) 3.0, allowing for fast and efficient charging across multiple voltage levels, including 5V, 9V, 12V, 15V, and 20V. This wide range of output voltages ensures compatibility with various medical devices, from small portable instruments to more substantial equipment. Additionally, the charger supports Qualcomm Quick Charge (QC) 4.0+ and QC 4.0 standards, delivering accelerated charging speeds and maximizing efficiency.
By utilizing Gallium Nitride (GaN) technology, EUM106AXR’s superior efficiency and thermal management capabilities allow the charger to operate at high performance levels while minimizing heat generation and energy loss. This design results in a compact, portable charger that doesn’t sacrifice power or reliability, making it ideal for space-constrained medical settings. The EUM106AS series is equipped with a universal input range of 100 to 240VAC, making it compatible with global power standards. This versatility is further enhanced by the charger’s support for multiple AC plug types, including options for the USA, EU, UK, SAA, China, South Africa, Korea, and India, allowing it to be used in virtually any region worldwide.
Safety is critical in medical environments, and the EUM106AXR series delivers with extensive protection mechanisms, including short circuit, over voltage, and over current protections. An optional over-temperature protection feature adds an extra layer of security, ensuring safe operation even in challenging conditions. These protections are essential for preventing damage to both the charger and the connected devices, ensuring safe and reliable power delivery. With no-load power consumption as low as ≤0.21W (Level VI) and ≤0.15W (CoC Tier 2), this charger is designed to minimize energy wastage, contributing to more sustainable energy use in medical facilities.
Customized solutions are available for GaN power series, please contact our sales team for your demands : sales@edac.com.tw
Product Highlights
- 2 x MOPP, IEC/EN60601-1 Compliance
- USB Power Delivery Function
- Gallium Nitride Based Design
- Protections : Short circuit / Over voltage / Over current
Over temperature (optional) - Energy Efficiency Level VI, CoC Tier II
- No Load Power Consumption ≤0.21W(VI) , ≤0.15W(CoC Tier II)
- MTBF > 100,000 hours
- P.D. 3.0 / QC 4.0+ / QC 4.0
- Customized Solutions Available

